USP-797 Compliance
USP-797 Compliant Cleanrooms
Modular cleanrooms can be designed as a single-pass cleanroom, a recirculating with air-chase walls and ceiling plenum, or as a hardwall partition for existing sterile environments.
On January 1, 2004 USP 797 regulations went into effect. These regulations are FDA enforceable and the FDA has indicated that it fully intends to do so.
USP 797 applies to healthcare personnel in all healthcare facilities where sterile preparations (CSP's) are compounded, stored, and dispensed, including community pharmacies, outsourcing pharmacies, physician practices, infusion clinics, surgical clinics, home care organizations, long-term care facilities and satellite pharmacies.
In order to be in compliance with the new rules, such institutions will needed to protect their products by utilizing a Laminar Airflow Workbench (LAFW's) within a cleanroom or, depending on requirements, a positive pressure barrier isolation which takes the place of a cleanroom by providing cleanroom conditions within a contained workspace.
By January 2005, the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) will require an action plan for each accredited healthcare organization that will bring the organization into compliance with USP 797 within a reasonable period of time.
Liberty offers a variety of solutions to enable compliance with the regulations no matter what the risk level.
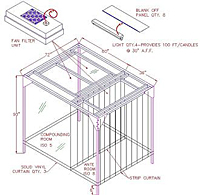
The accompanying diagram on the right features a Class 5 cleanroom with an accompanying class 8 anteroom which provides a clean area for donning gloves, gowns, etc. with a total footprint of 6' x 8'.
Unit is supplied with lights, strip curtains and a fan filter unit.
Liberty's barrier isolator, pictured at the link above, isolates areas from various contamination sources including the volume of air surrounding the equipment and the space from ceiling to floor. A barrier isolator encompasses a much smaller area around the process allowing a small volume and proper airflow and totally removes workers contamination from the process.
Whether the decision is to install a cleanroom or opt for a barrier isolators, the primary goal is safety. Both systems have their benefits and drawbacks, but it is up to the individual pharmacy directors to make the best decision for their business, their personnel and their patients.
On January 1, 2004 USP 797 regulations went into effect. These regulations are FDA enforceable and the FDA has indicated that it fully intends to do so.
USP 797 applies to healthcare personnel in all healthcare facilities where sterile preparations (CSP's) are compounded, stored, and dispensed, including community pharmacies, outsourcing pharmacies, physician practices, infusion clinics, surgical clinics, home care organizations, long-term care facilities and satellite pharmacies.
In order to be in compliance with the new rules, such institutions will needed to protect their products by utilizing a Laminar Airflow Workbench (LAFW's) within a cleanroom or, depending on requirements, a positive pressure barrier isolation which takes the place of a cleanroom by providing cleanroom conditions within a contained workspace.
By January 2005, the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) will require an action plan for each accredited healthcare organization that will bring the organization into compliance with USP 797 within a reasonable period of time.
Liberty offers a variety of solutions to enable compliance with the regulations no matter what the risk level.
The accompanying diagram on the right features a Class 5 cleanroom with an accompanying class 8 anteroom which provides a clean area for donning gloves, gowns, etc. with a total footprint of 6' x 8'.
Unit is supplied with lights, strip curtains and a fan filter unit.
Liberty's barrier isolator, pictured at the link above, isolates areas from various contamination sources including the volume of air surrounding the equipment and the space from ceiling to floor. A barrier isolator encompasses a much smaller area around the process allowing a small volume and proper airflow and totally removes workers contamination from the process.
Whether the decision is to install a cleanroom or opt for a barrier isolators, the primary goal is safety. Both systems have their benefits and drawbacks, but it is up to the individual pharmacy directors to make the best decision for their business, their personnel and their patients.
Unit of Measure